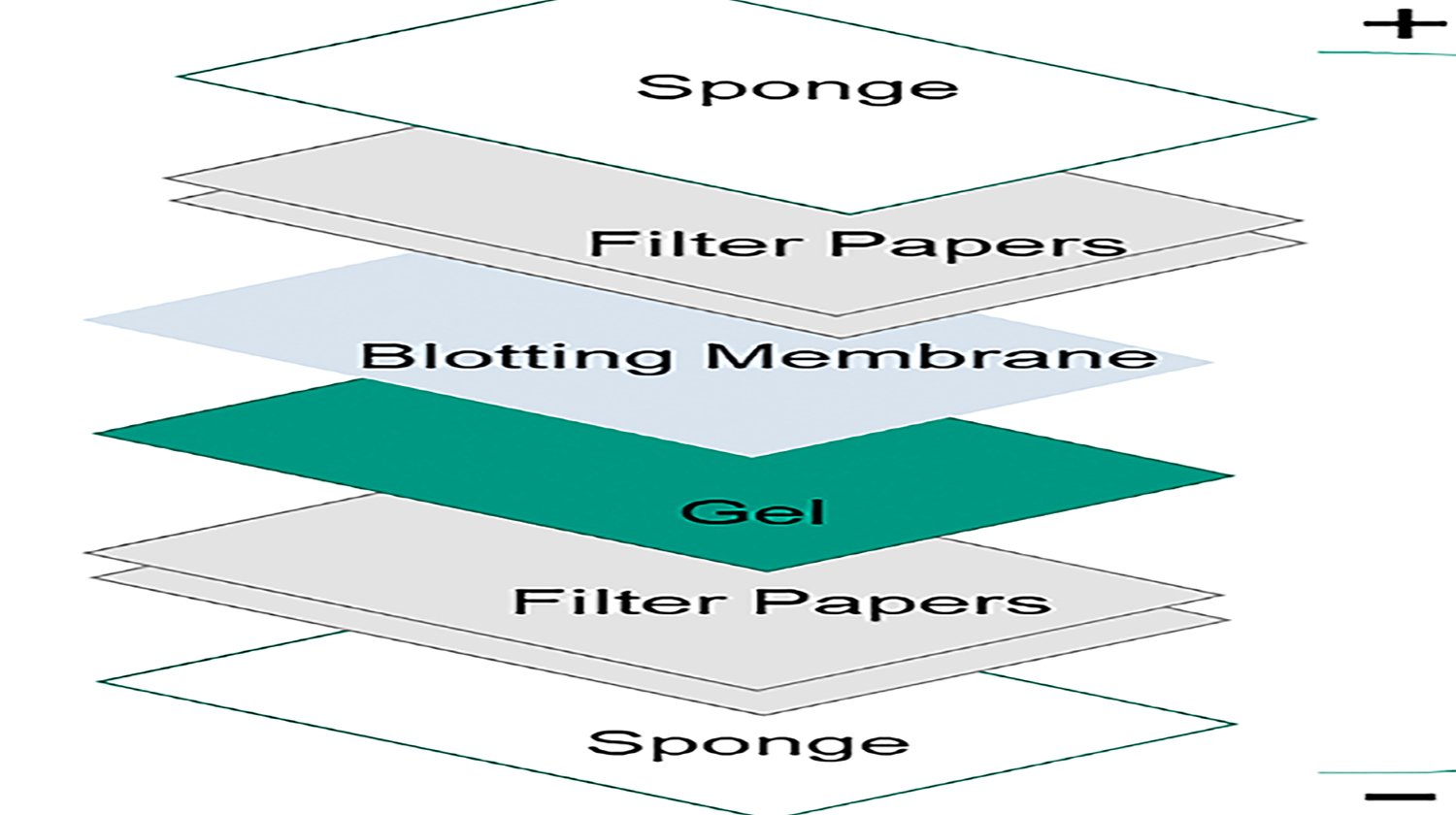
Choosing an appropriate membrane for your western blotting (WB) is critical. Among many choices, the most favorable membranes are polyvinylidene difluoride (PVDF) and nitrocellulose (NC), as both possess several vital attributes that make them suitable for Western blot conditions. Although a researcher sometimes grabs whatever is available for a WB assay, we should know that PVDF and NC membranes are not always exchangeable. The properties of your protein of interest and the downstream detection steps required in your application will determine which membrane can give you the expected results. In detail, you need to consider the following factors before you choose which membrane to use.

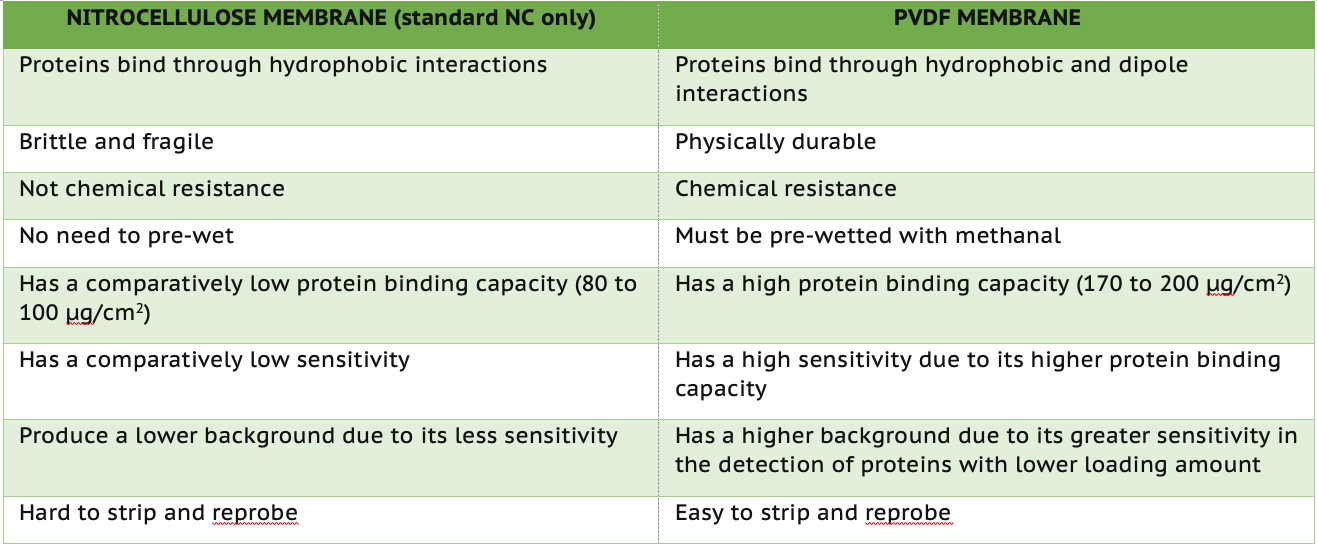
1. Protein Binding Capacity.
PVDF has a protein binding capacity of 170 to 200 μg/cm2, while NC has a protein binding capacity of 80 to 100 μg/cm2. Since PVDF has a higher protein binding capacity, it also offers higher sensitivity. While this feature allows it to detect lowly expressed proteins, you are also more likely to get higher background noise in your antibody detection steps. On the other hand, the NC membrane may not be capable of the detection sensitivity of PVDF membranes but will produce lower background noise.
2. Binding Interactions.
Protein molecules bind to NC membranes through hydrophobic interactions, while molecules bind to PVDF membranes through hydrophobic and dipole interactions.
3. Physical Characteristics.
Standard NC membrane is brittle and fragile, while PVDF is more durable and has higher chemical resistance. If a detection needs to strip and reprobe, PVDF is ideal for use compared to standard NC.
4. Pore Size.
Both membranes have typical pore sizes of 0.1, 0.2, or 0.45μm. The 0.45μm membrane is suitable for most protein blotting applications. But for smaller peptides or lower molecular weight proteins (less than 20 kDa), you should use a 0.1 or 0.2μm pore size membrane. Please be aware that when detecting a protein loaded at low levels or when quantification is considered critical, you should always choose a smaller membrane like 0.2μm.
5. Transfer Buffer Components.
Using methanol in the transfer buffer reduces the gel's pore size and worsens high-molecular-weight (HMW) protein mobility. In addition, 10~20% methanol may cause HMW protein precipitation, trapping protein in the gel. Together will yield a poor transfer result with HMW proteins, which are membrane independent. Some discussions suggest PVDF for the HMW protein transfer without context. Please note that if the regular methanol-containing transfer buffer is used and PVDF shows a better WB result, it is very likely attributed to its higher protein binding capacity. Many studies have reported that a low concentration detergent such as SDS or Tween-20 in the transfer buffer could dramatically increase the transfer efficiency of HMW proteins on both PVDF and NC membranes.
6. Membrane Format.
Commercially available membranes may have different formats. Several considerations must be taken into account when choosing the most suitable membrane format, including transfer system (semi-dry, wet, or fast), detection protocols, convenience, and flexibility. For instance, if convenience, reproducibility, and high throughput are of your highest importance, pre-cut and pre-wetted membranes are the ideal choice. If your gel sizes are variable, rolls of membrane offer you the flexibility to cut the membrane with a particular size to match your gel. Suppose extra stripping and reprobing are required in your detection, and you are also concerned about high background noise. In that case, the supported NC membrane could be considered more durable and resilient than standard NC membranes while maintaining other properties of standard NC.
While both NC and PVDF membranes are used for Western blotting, it is critical to understand the key features of each more extensively. Here, we summarized our discussion into a table, providing a guide to help your experimental design. Kindly note that multiple testing must be conducted to determine the optimized choice due to the complexity of biology (diversity of biological samples and the complexity of assay conditions).