The recent emergence of SARS-CoV-2 variants has prompted concerns as to their susceptibility to vaccine neutralization. Meanwhile, another concern on whether such strains may evade detection by diagnostics draws equal attention because this possibility will dampen our accuracy of disease tracking.
---------------------------------------------------------------------------------------------------------------------------------
-----------------------------------------------------------------------------------------------------------------------------
As the spike protein (Spike or S protein for short) contains sequences unique to SARS-CoV-2, detection on S protein potentially minimizes cross-reactivity to sequences present in other known human coronaviruses, such as SARS-CoV, MERS, and human coronaviruses 229E, OC43, HKU-1, and NL63. Therefore, S protein has been utilized as the leading target in diagnostic tests, epidemiological studies, and vaccine and drug development since the outbreak of the severe pandemic caused by SARS-CoV-2 infection. Impressively, currently deployed vaccines against COVID-19 are all based on S protein, including traditional inactivated vaccines, recombinant protein vaccines, adenovirus vector vaccines or mRNA vaccines. Although the known mutations in S protein are reported not to completely abandon the effects of currently deployed vaccines, concerns on antibody-based COVID-19 in vitro diagnostic tests become more and more serious because even if the changes in the viral protein sequences may only alleviate the antibody binding, the rate of false-negative results will significantly increase due to the compromised testing sensitivity and accuracy.
----------------------------------------------------------------------------------------------------------------------------
-----------------------------------------------------------------------------------------------------------------------------
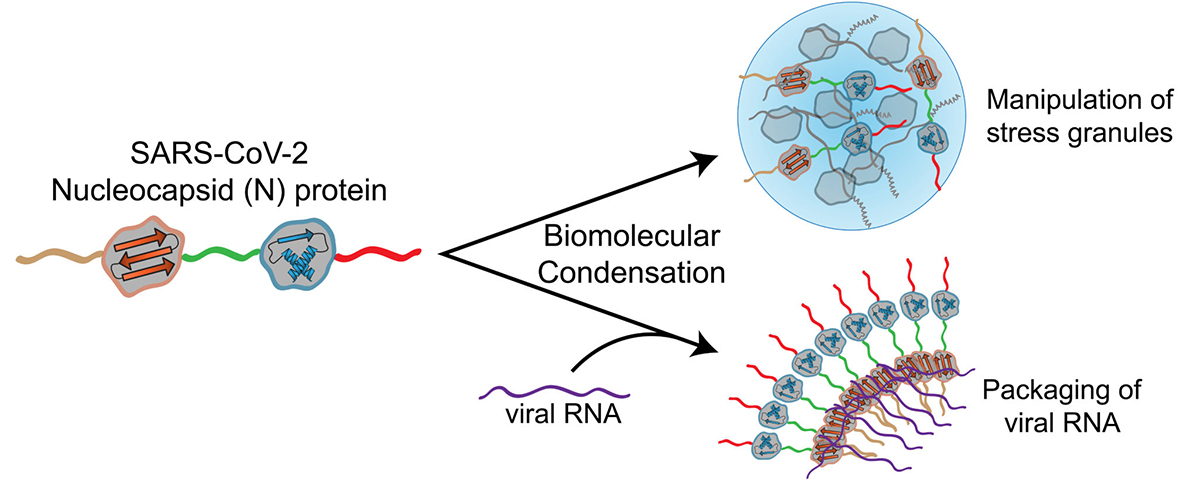
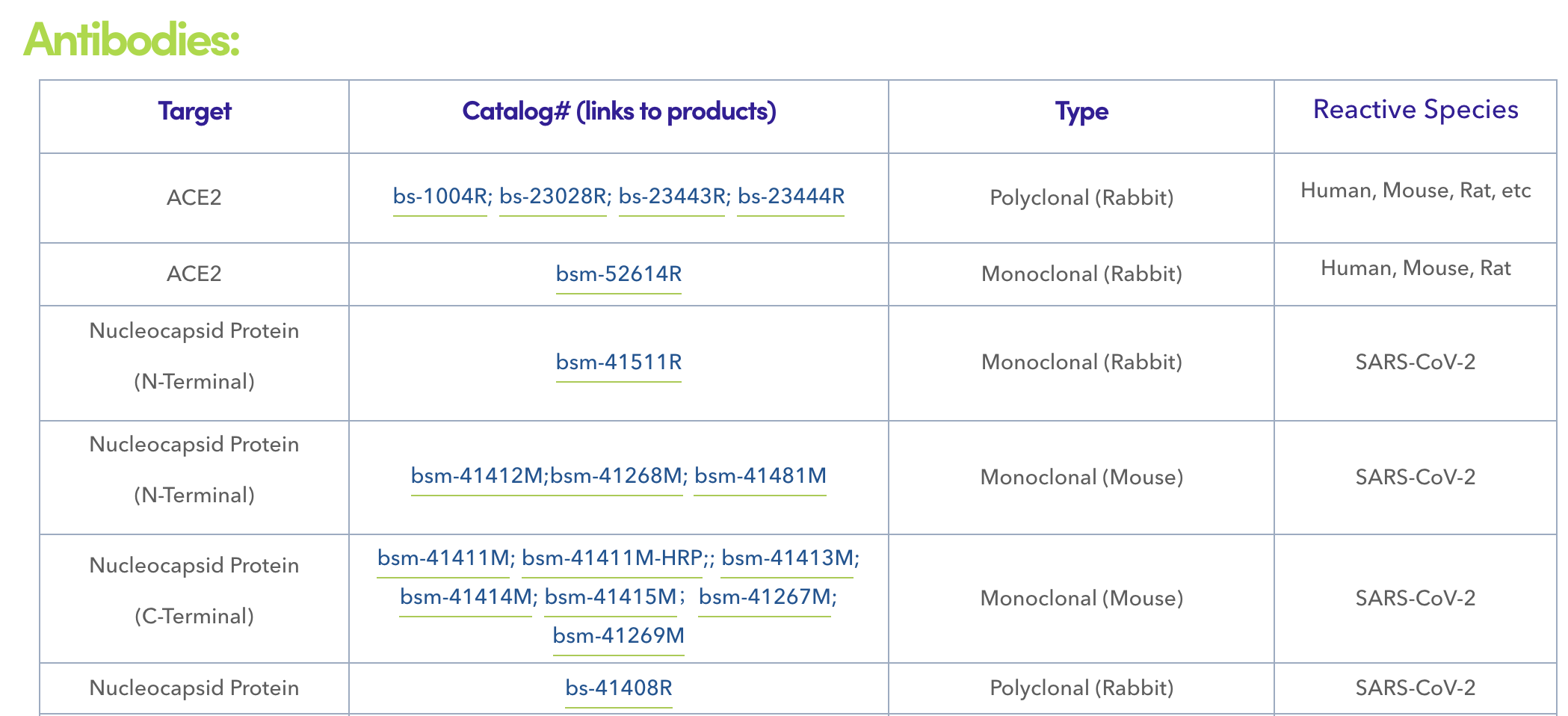
ELISAs (enzyme-linked immunosorbent assays) and LFAs (lateral flow assays) are the two most popular antibody-based immunoassays for the detection of SARS-CoV-2 viral proteins. The targeted analytes in these assays are predominantly S or nucleocapsid (N) proteins, the two most abundant and immunogenic viral proteins present in the SARS-CoV-2 genome. At present, emerging evidence indicated that S protein is the most likely viral protein to undergo mutation, and many mutations have been characterized to affect viral function, including infection rate, transmissibility, and the ability to infect individuals younger in age (for example, a mutation near the receptor binding domain may affect entry into the host cell). With this in mind, diagnostic tests designed to target S protein using monoclonal antibodies must revalidate the performance of the test against emerging strains of SARS-CoV-2. Conversely, N protein shows an interesting property, i.e., point mutations in the N protein are less likely to occur and less likely to affect viral function. This feature is beneficial to help obtain sufficiently accurate testing results to detect the virus, and therefore, N protein is considered the better target for in vitro diagnostic detection for COVID-19.
------------------------------------------------------------------------------------------------------------------------------
------------------------------------------------------------------------------------------------------------------------------
Bioss is a leading manufacturer of antibodies and recombinant proteins in support of scientific research and the development of in vitro diagnostics. The company employs an application-oriented development strategy, with a particular focus on product design, quality control, and solution-based support. In response to the ongoing pandemic, Bioss has developed SARS-CoV-2 antigens and antibodies specifically designed and optimized for in vitro diagnostics, including a serial of monoclonal antibodies recognizing different epitopes of N protein.