Fluorescent vs. Chromogenic Detection in Immunohistochemistry
Antibody detection for IHC typically occurs through either a fluorescent or chromogenic reaction. While the goal of each technique is similar — to label targets with high spatial resolution and low background — both methods have benefits and pitfalls.
Fluorescent labeling relies on conjugation of the primary or secondary antibody with a fluorophore or other molecule. These fluorescent molecules emit light within specific ranges between 300 and 800 nm when excited by a light source. Given the full range of emission wavelengths within the useable spectra, multiple fluorescent markers can be used simultaneously for detailed co-localization characterizations.
For direct detection, the fluorophore is conjugated to the primary antibody. For indirect detection, the fluorophore is conjugated to the secondary antibody.
View excitation and emission ranges for Bioss products
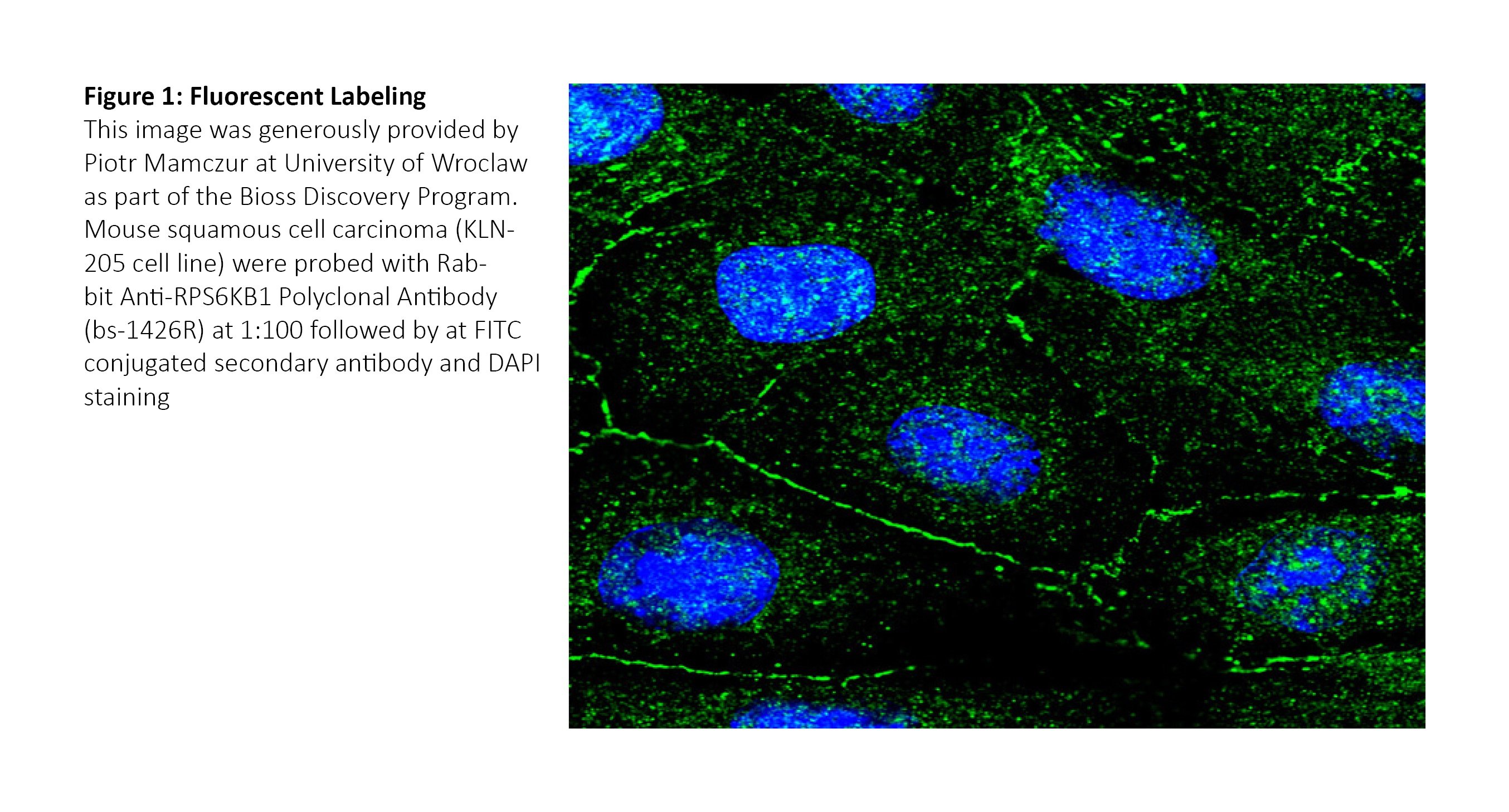
Chromogenic labeling relies on chemical reactions triggered by enzymes conjugated with either the primary or secondary antibody. Peroxidases such as HRP are a commonly used tool for such reactions.
Once added, liquid substrate reacts with these enzymes and forms a solid, color marking at the target site. These markings can be viewed through standard microscopy techniques and do not require excitation by a light source.
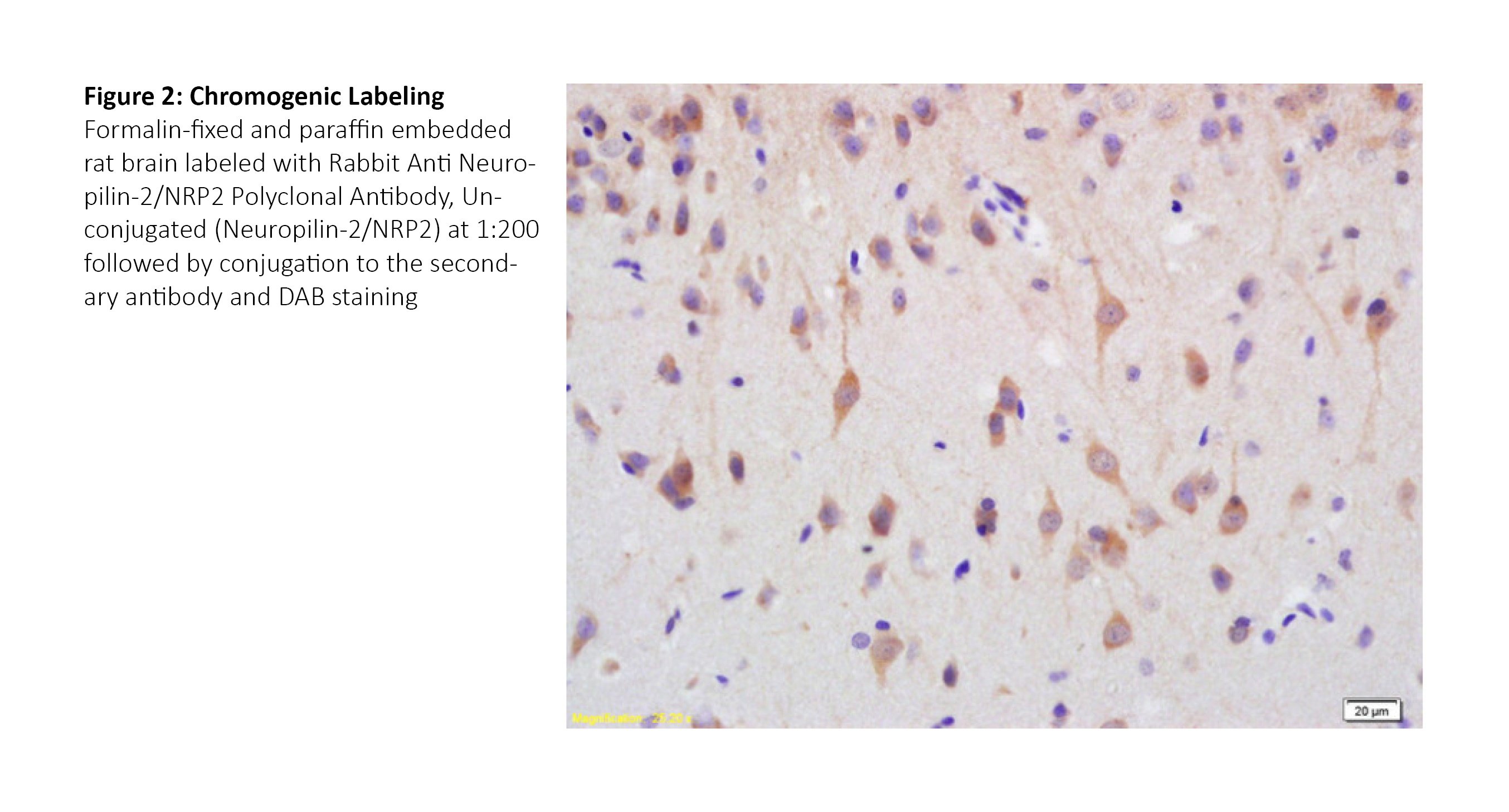
View more resources for immunohistochemistry
Considerations for selecting a chromogenic or fluorescent detection method:
How abundant is your protein of interest?
Generally speaking, chromogenic techniques will provide higher sensitivity when paired with appropriate signal amplification protocols. These can include incubation with either avidin-biotin complex (ABC) or labeled streptavidin-biotin (LSAB), which can both increase signal several fold versus a labeled secondary antibody.
Do you need to label more than two targets?
Based on the limited selection of chromogenic reagents, fluorescent markers are superior for detecting multiple signals from the same tissue sample. Fluorescent markers can be manipulated by changing the excitation wavelength(s) and intensity(s), and spectra can be analyzed before staining for potential overlap. Overlapping chromogenic stains, in contrast, can yield confusing or misleading results.
Do you need to identify any co-localized targets?
Fluorescent markers are better suited for testing co-localization of two or more target proteins in the same cell structures. Whereas chromogenic stains will either blend or overlap, fluorescent markers can be analyzed independently. Overlaying multiple fluorescent images from the same tissue sample can provide a complete picture of protein expression and interactions.
Do you need to preserve samples for an extended time?
Under normal conditions, fluorescent labels are more prone to photobleaching, which over time will result in a diminished signal. Since chromogenic reactions rely on a substrate changing from liquid to solid state, the labeling is more durable. However, both methods require careful storage and limited light exposure between imaging sessions for best preservation.
Are you imaging in the liver or kidney, or using frozen sections?
This suggestion is a bit more specific: Endogenous biotin in the liver or kidney (or in frozen tissue sections) is known to react with some signal amplification techniques, causing high background. If working in any of these conditions, it may be necessary to alter your protocol or convert to fluorescent detection. In any case, it’s vital to run proper isotype controls to determine background biotin levels when using ABC or LSAB incubations.
