COVID-19 pandemic was caused by the novel SARS-CoV-2 virus (Severe acute respiratory syndrome coronavirus 2). SARS-CoV-2 enters target cells through the interaction of its envelope spike protein with the primary host cell receptor ACE2 (angiotensin-converting enzyme-2), which is then cleaved by a serine protease (TMPRSS2) to allow viral fusion and entry across the cell membrane. Antibodies capable of binding to the spike protein can neutralize viral entry into cells and are considered to play an important role in the protective immune response to SARS-CoV-2 infection.
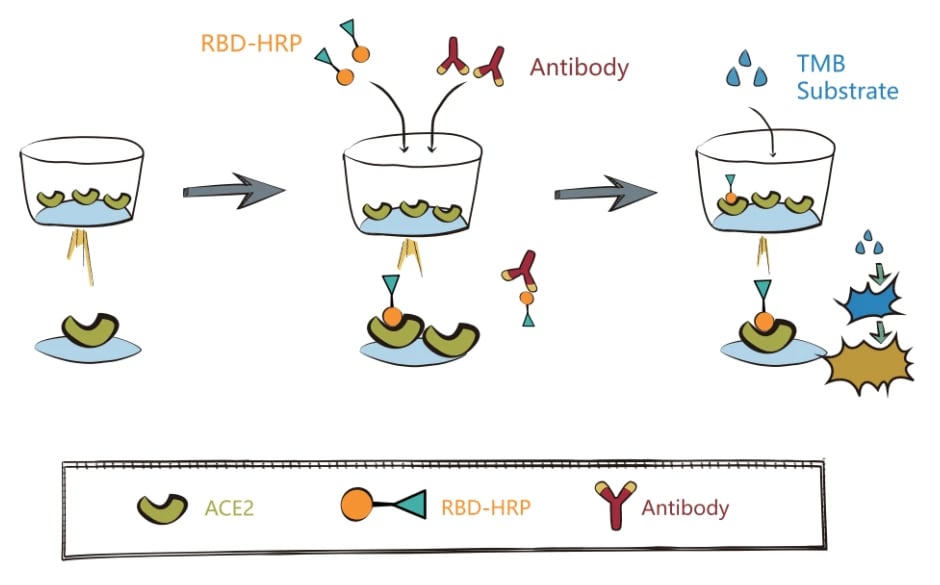
The efficacy of most licensed vaccines correlates with the antigen-neutralizing antibodies elicited by vaccination. With the COVID-19 vaccine administration, it is critical to detect and measure the level of neutralizing antibodies in vaccinated people to address the vaccination's protective effect. Currently, the most convenient detection for SARS-CoV-2 neutralization antibodies is to check if the interaction of ACE2 and RBD (receptor binding domain) of spike proteins was inhibited. Briefly, plasma samples containing SARS-CoV-2 neutralization antibodies are used to compete with ACE2 for RBD binding in vitro. The percent binding inhibition between ACE2 and RBD will be interpreted as percent neutralization, indicating the vaccination effect.
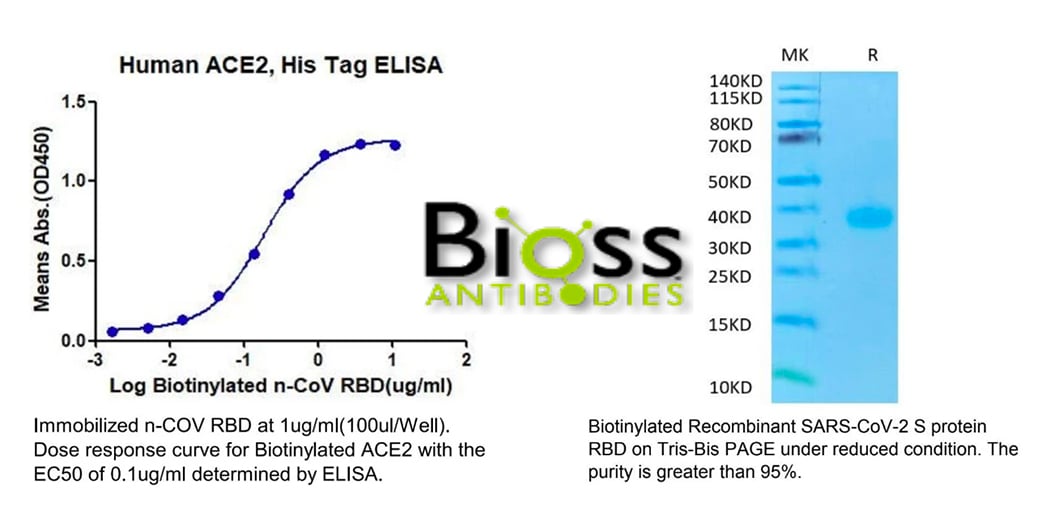
The assay principle is straightforward, but it is conceivable that the accurate results must rely on many high-quality reagents, such as the robust ACE2 and RBD proteins and the positive control, SARS-CoV-2 specific antibodies. Thankfully, in support of the fighting against the COVID-19 disease, many organizations were dedicated to developing a variety of SARS-CoV-2-related proteins and antibodies. On the basis of their contribution, some reliable assay kits for the detection of SARS-CoV-2 neutralization antibodies are available on the market for both RUO and IVDpurposes.
____________________________________
REFERENCES:
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-test-detects-neutralizing-antibodies-recent-or